KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
$194.99
Shipping Calculated at checkout.
Availability: 258 in stock
FREE SHIPPING
99%+ PURITY
MADE IN USA
Vial
- Mazdutide 10MG
- AICAR 50MG
- Lipo-C 10ML
- Survodutide 10MG
- LC-526 Metabolic Complex
- SSJ-9 Amino Matrix
- Epitalon
- L-Carnitine 600MG / 10ML
- Cerebrolysin 60MG
- Oxytocin 5mg
- SELANK
- 5-Amino-1MQ
- VIP
- L-Glutathione 1500MG
- Acetic Acid Solution
- HCG 10000iu
- BAC Water 30ML
- BAC Water 10ML
- GLOW 70MG
- IGF-1 LR3 1MG
- Thymalin
- HCG (5000iu)
- Wolverine Stack Peptides – BPC-157 (5mg) / TB-500 (5mg)
- BAC Water 3ML
- Melanotan II (MT-2) 10MG
- SLU-PP-332 (5MG)
- KPV 10MG
- GHK-Cu
- KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
- Gonadorelin
- L-Glutathione (200MG)
- SEMAX (10MG)
- Cagrilintide (10MG)
- DSIP – Delta Sleep‑Inducing Peptide (5MG)
- RETA GLP-3
- Kisspeptin-10
- Ipamorelin / CJC-1295 No Dac 10mg
- Tirz GLP-2
- L-Glutathione (500mg)
- Pancragen 20 MG
- Prostamax 20 MG
- Livagen 20 MG
- Testagen 20 MG
- CardioCytogen 20 MG
- TB-4
- ARA‑290 10mg
- FOXO4-DRI (D-Retro-Inverso) 10mg
- SS-31 (10MG)
- Tesamorelin (10MG)
- AOD-9604
- IPAMORELIN 10MG
- PT-141 10MG
- NAD+
- TB-500 10MG
- SEMA GLP-1
- MOTS-c 10MG
- CJC-1295 (10MG)
- BPC-157 (10MG)
IRON
- Phenibut (60 capsules)
- NAD+ 500MG Spray
- Mazdutide 10MG
- AICAR 50MG
- Lipo-C 10ML
- Survodutide 10MG
- Methylene Blue 20MG
- 5-Amino-1MQ Capsules 50mg 60 cap
- LC-526 Metabolic Complex
- SSJ-9 Amino Matrix
- MITOCHONDRIAL POWER STACK
- SHRED MATRIX X4
- ANABOLIC SIGNALING MATRIX X3
- L-Carnitine 600MG / 10ML
- Cerebrolysin 60MG
- Oxytocin 5mg
- SELANK
- 5-Amino-1MQ
- VIP
- L-Glutathione 1500MG
- Acetic Acid Solution
- HCG 10000iu
- BAC Water 30ML
- BAC Water 10ML
- GLOW 70MG
- IGF-1 LR3 1MG
- Thymalin
- HCG (5000iu)
- Wolverine Stack Peptides – BPC-157 (5mg) / TB-500 (5mg)
- BAC Water 3ML
- Melanotan II (MT-2) 10MG
- SLU-PP-332 (5MG)
- KPV 10MG
- GHK-Cu
- KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
- Gonadorelin
- L-Glutathione (200MG)
- SEMAX (10MG)
- Cagrilintide (10MG)
- DSIP – Delta Sleep‑Inducing Peptide (5MG)
- RETA GLP-3
- MK-ULTRA (MK-777)
- Kisspeptin-10
- VIP 10MG Spray
- Ipamorelin / CJC-1295 No Dac 10mg
- Tirz GLP-2
- L-Glutathione (500mg)
- N-Acetyl Selank Spray
- N-acetyl Semax Spray
- Adamax Nootropic – Nasal Spray
- Adalank Nootropic Peptide – Nasal Spray
- Pancragen 20 MG
- Prostamax 20 MG
- Livagen 20 MG
- Testagen 20 MG
- CardioCytogen 20 MG
- TB-4
- ARA‑290 10mg
- FOXO4-DRI (D-Retro-Inverso) 10mg
- KPV 250 MCG (60 Capsules)
- SLU-PP-332 (60 capsules, 250MCG)
- SS-31 (10MG)
- Tesamorelin (10MG)
- MK-677 12.5MG (60 capsules)
- AOD-9604
- IPAMORELIN 10MG
- Healing and Repair Research Blend (60 capsules)
- BPC-157 250MCG (60 capsules)
- Tesofensine 500MCG (100 capsules)
- PT-141 10MG
- NAD+
- TB-500 10MG
- SEMA GLP-1
- MOTS-c 10MG
- CJC-1295 (10MG)
- BPC-157 (10MG)

What is KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)?
Introduction
KLOW-80 is a pre-measured, research-grade peptide blend formulated for laboratory and experimental research purposes, consisting of four well-characterised synthetic peptides: GHK-Cu, BPC-157, TB-500 (a fragment of Thymosin β4), and KPV. Each component is included in standardized proportions to allow consistency and reproducibility in research settings, where these peptides are commonly studied for their biochemical roles, cellular signaling properties, and interactions within experimental models. Although BPC-157, TB-500, GHK-Cu, and KPV are frequently associated with similar biological processes such as cellular resilience, modulation of inflammatory responses, and tissue repair signaling, each peptide operates via its own distinct molecular and intracellular pathways. Integrating these peptides into a unified formulation allows investigators to explore how parallel yet non-identical mechanisms interact and collectively influence repair dynamics, structural integrity of tissues, and sustained functional performance over time. The formulation was specifically designed to streamline research workflows by minimizing the operational burden of preparing and delivering multiple agents separately. This consolidated approach enables researchers to allocate greater attention to pathway analysis, molecular markers, and experimental endpoints, while also promoting consistency across study conditions and improving overall experimental efficiency. BPC-157 is a laboratory-synthesized peptide composed of fifteen amino acids that has been widely investigated in experimental models focused on tissue damage and structural recovery. In tendon-focused research settings, it has been associated with cellular behaviors relevant to tissue organization, including support of cell survival, directional movement, and coordinated cellular arrangement within compromised connective matrices. Observations from controlled studies suggest involvement in fibroblast-associated mechanisms, contributing to matrix interaction and cellular expansion during phases of tissue reorganization. Further preclinical research has explored its relationship with molecular pathways linked to vascular regulation, nitric oxide signaling, and cellular protection, all of which are pertinent to connective tissue research contexts. As a result, BPC-157 is commonly examined within regenerative biology and mechanobiology models to better understand tendon structure, cellular responses to mechanical loading, and peptide driven signaling under laboratory conditions without implication of clinical or therapeutic use. Prezatide copper, commonly known as GHK-Cu, is a synthetically produced copper binding tripeptide widely examined in biochemical and dermatological research environments. It consists of a short amino-acid sequence complexed with copper ions, a structure that contributes to its stability and interaction with cellular systems under laboratory conditions. Owing to its well defined molecular profile, Prezatide copper has been frequently utilized in experimental studies exploring peptide metal interactions, cellular communication, and protein regulatory mechanisms. In research settings, this compound has been investigated for its involvement in processes related to cellular maintenance, matrix dynamics, and signaling pathways associated with tissue structure. Its role in experimental models often centers on understanding how copper peptide complexes influence cellular activity, gene expression patterns, and extracellular matrix behavior. As such, Prezatide copper remains primarily a subject of laboratory inquiry and analytical study, rather than a clinically applied substance. Timbetasin is a synthetic peptide derived from thymic peptides, characterized by a defined amino acid sequence that underpins its unique biochemical and structural properties. Its molecular composition allows for specific interactions with cellular proteins and signaling pathways, making it a valuable tool in experimental studies of tissue organization, cellular migration, and cytoskeletal regulation. The peptide’s stability, solubility, and capacity to form transient complexes with intracellular targets are key biochemical features that influence its behavior in laboratory settings. Structurally, it exhibits conformational properties that facilitate its interaction with receptor sites and other biomolecules, enabling it to modulate cellular activities in controlled experimental models. Its tertiary and secondary structural elements contribute to peptide folding, molecular recognition, and signaling efficacy, which are essential for understanding its mechanistic roles in cellular and tissue-level processes. These characteristics make Timbetasin an important research compound for exploring peptide-mediated regulation without implying therapeutic or clinical applications. KPV is a short tripeptide (Lys-Pro-Val) derived from the C-terminal segment of α-melanocyte-stimulating hormone (α-MSH), a melanocortin peptide involved in immune and inflammatory regulation. Despite its small molecular size, it retains functional elements of the parent hormone and has been investigated in experimental systems for its influence on inflammatory signaling pathways and cellular stress responses. Evidence from laboratory and animal studies indicates that this fragment contributes to the anti-inflammatory properties associated with α-MSH, supporting modulation of cytokine activity and immune-cell behavior in controlled research environments . Experimental findings further suggest that KPV may regulate inflammatory cascades by influencing transcriptional mediators such as NF-κB and by altering the production of pro-inflammatory cytokines and adhesion molecules. It has also been explored for its interaction with peptide transport systems and epithelial cellular pathways, which may contribute to barrier protection and localized immune modulation in inflamed tissues. These biochemical characteristics position KPV as a frequently studied component in peptide-based research models examining immune balance, epithelial stability, and inflammation-associated cellular signaling.
Chemical Structure of KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
GHK-CU
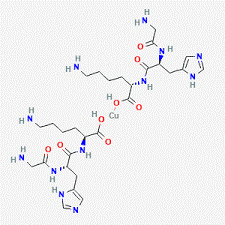
KPV
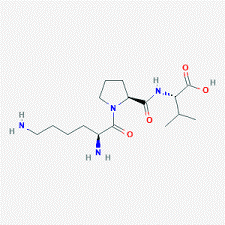
BPC-157
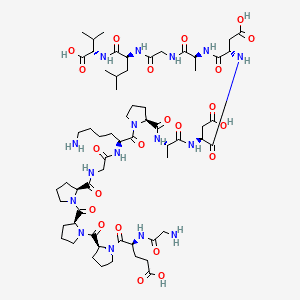
Image Source: https://pubmed.ncbi.nlm.nih.gov/
| Molecular Formula | · Bpc-157: C62HggN16022
· Prezatide copper: C14H23CuN604+ · Timbetasin: C212H350N56078S |
| Molecular Weight
|
· Bpc-157: 1419.5
· Prezatide copper: 402.92 · Timbetasin: 4963 |
| Onoisotopic Mass
|
· Bpc-157: 1418.70415882
· Prezatide copper: 402.107675 · Timbetasin: 4960.4863169 |
| Polar Area
|
· Bpc-157: 573
· Prezatide copper: 179 · Timbetasin: 2250 |
| Complexity
|
· Bpc-157: 3040
· Prezatide copper: 428 · Timbetasin: 12200 |
| XLogP
|
· Bpc-157: -9
· Timbetasin: -43.5 |
| Heavy Atom Count
|
· Bpc-157: 100
· Prezatide copper: 25 · Timbetasin: 347 |
| Hydrogen Bond Donor Count
|
· Bpc-157: 16
· Prezatide copper: 5 · Timbetasin: 72 |
| Hydrogen Bond Acceptor count | · Bpc-157: 24
· Prezatide copper: 7 · Timbetasin: 88 |
| Rotatable Bond | · Bpc-157: 39
· Prezatide copper: 10 · Timbetasin: 180 |
| Pubchem LCSS | · Bpc-157: laboratory chemical safety summary
· Prezatide copper: chemical safety summary · Timbetasin: chemical safety summary |
Identifiers
| CID | · Bpc-157: 9941957
· Prezatide copper: 71587328 · Timbetasin: 16132341 |
| InChl | Bpc-157:
InChl=1S/C62H98N16022/c1-31(2)25-37(55(92)74-50(32(3)4)62(99)100)71-46(81)29-65-51(88)33(5)67-53(90)38(26-48(84) 85)73-54 (91)39(27-49(86)87)72-52(89)34(6)68-57(94)41-15-10-21-75(41)58(95)35(13-7-8-20-63)70-45(80)30-66-56(93)40-14-9-22-76(40)60(97)43-17-12-24-78(43)61(98)42-16-11-23-77(42)59(96)36(18-19-47(82)83)69-44(79)28-64/h31-43,50H,7-30,63-64H2, 1-6H3, (H,65,88)(H,66,93)(H,67,90)(H,68,94)(H,69,79)(H,70,80) (H,71,81)(H,72,89)(H,73,91)(H,74,92)(H,82,83)(H,84,85) (H,86,87)(H,99,100)/133-,34-, 35-, 36-,37-, 38-,39-,40-, 41-,42-,43-, 50-/m0/s1 Prezatide copper: InChl=1S/C14H24N604.Cu/c15-4-2-1-3-10(14(23)24)20-13(22)11(19-12(21)6-16)5-9-7-17-8-18-9;/h7-8, 10-11H, 1-6, 15-16H2, (H, 17,18)(H,19,21) (H,20,22) (H,23,24);/q;+2/p-1/t10-, 11-;/m0./s1 Timbetasin: InChl=1S/C212H350N56078S/c1-16-106(7)166(261-190(323)131(64-75-160(292)293)231 172(305)109(10)228-174(307)134(78-91-347-15)245-196(329)140(98-165(302)303\255- 201(334) 147-53-39-88-266(147)209(342)135(52-29-38-87-221)250-197(330)139(97-164(300)301)254-198(331)143(100-269)229-113(14)276)204(337)246-129(62-73-158(288)289)187(320)233-118(47-24-33-82-216)179(312)252-137(94-114-42-19-18-20-43-114)194(327)253-138(96-163(298)299)195(328)237-121(50-27-36-85-219)181(314)258-144(101-270)199(332)239-119(48-25-34-83-217)178(311)251-136(92-104(3)4)193(326)236-116(45-22-31-80-214)175(308)235-122(51-28-37-86-220)189(322)263-168(110(11)273)207(340)249-133(66-77-162(296)297)192(325)264-169(111(12)274)206(339)248-126(58-69-152(224)279)184(317)243-128(61-72-157(286)287)186(319)234-120(49-26-35-84-218)180(313)256-142(95-153(225)280)211(344)268-90-40-54-148(268)202(335)257-141(93-105(5)6)210(343)267-89-41-55-149(267)203(336)259-145(102-271)200(333)238-117(46-23-32-81-215)177(310)244-132(65-76-161(294)295)191(324)265-170(112(13)275)208(341)262-167(107(8)17-2)205(338)247-130(6374-159(290)291)188(321)241-125(57-68-151(223)278)183(316)242-127(60-71-156(284)285)185(318)232-115(44-21-30-79-213)176(309)240-124(56-67-150(222)277)173(306)227-108(9)171(304)226-99-154(281)230-123(59-70-155(282)283)182(315)260-146(103-272)212(345)346/h18-20,42-43, 104-112,115-149,166-170,269-275H, 16-17,21-41,44-103,213-221H2,1-15H3, (H2,222,277)(H2,223,278) (H2,224,279)(H2,225,280) (H,226,304)(H,227,306) (H,228,307)(H,229,276) (H,230,281)(H,231,305) (H,232,318)(H,233,320) (H,234,319)(H,235,308) (H,236,326)(H,237,328) (H,238,333)(H,239,332) (H,240,309)(H,241,321) (H,242,316)(H,243,317) (H,244,310)(H,245,329) (H,246,337)(H,247,338) (H,248,339)(H,249,340) (H,250,330)(H,251,311) (H,252,312)(H,253,327) (H,254,331)(H,255,334) (H,256,313)(H,257,335) (H,258,314)(H,259,336) (H,260,315)(H,261,323) (H,262,341)(H,263,322) (H,264,325)(H,265,324) (H,282,283)(H,284,285) (H,286,287)(H,288,289) (H,294,295)(H,296,297)(H,298,299)(H,300,301)(H,302,303)(H,345,346)/t106-, 107-, 108-, 109-, 1 10+, 111+, 112+, 115-, 116-, 117-, 118-, 119-, 120-, 121-, 122-, 123-, 124-, 125-, 126-, 127-, 128-, 129-, 130-, 131-, 132-, 133-, 134-, 135-, 136-, 137-, 138-, 139-, 140-, 141-, 142-, 143-, 144-, 145-,146-, 147-, 148-, 149-, 166-, 167-, 168-, 169-, 170-/m0/s1 |
| ChiKey
|
Bpc-157: HEEWEZGQMLZMFE-RKGINYAYSA-N
Prezatide copper: NZWIFMYRRCMYMN-ACMTZBLWSA-M Timbetasin: UGPMCIBIHRSCBV-XNBOLLIBSA-N
|
| Isometric SMILES | Bpc-157:
C[C@@H](C(=O)N[C@@H](CC(=O)O)C(=O)N[C@@H](CC(=0)O)C(=0)N[C@@H](C)C(=O)NCC(=0)N [C@@H](CC(C)C)C(=O)N[C@@H] (C(C)C)C(=0)O)NC(=0)[C@@H]1CCCN1C(=O)[C@H](CCCCN)NC (=O)CNC(=0)[C@@H|2CCCN2C(=O)[C@@H]3CCCN3C (=0)[C@@H]4CCCN4C(=O)[C@H](CCC(=0)O)NC(=0)CN Prezatide copper: C1=C(NC=N1)C[C@@H](C(=O)N[C@@H](CCCCN)C(=O)[O-])NC(=O)CN.[Cu+2] Timbetasin: CC[C@H](C)[C@@H](C(=O)N[C@@H](CCC(=0)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC1=CC=CC=C1)C(=O)N[C@@ H](CC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(FO)N[C@@H]([C@@H](C)O)C(=0)N[C@@H](CCC(=0)O)C(=O)N[C@@H]([C@@H](C)O)C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](CCC(=0)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H] (CC(=O)N)C(=O)N2CCC[C@H|2C(O)NIC@@H] (CC(C)C)C (=O)N3CCC[C@HJ3C(=O)N[C@@H](CO)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(=0)O)C(=O)N[C@@H]([C@@H](C)O)C(=0)N[C@@H]([C@@H](C)CC)C(=0)N[С@@Н](CCC(=0)O)C(=O)N[C@@H](CCC(=0)N)C(=O)N[C@@H](CCC(=O)O)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCC(=O)N)C(=O)N[C@@H](C)C(=O)NCC(=O)N[C@@H](CCC(=O)O)C(=O)N[С@@H](CO)C(=O)O)NC(=O)[С@H](CCC(=O)O)NC(=О)[С@H](C)NC(=O)[C@H](CCSC)NC(=0)[C@H](CC(=0)O)NC(=0)[C@@H]4CCCN4C(=O)[С@H](CCCCN)NC(=O)[C@H](CC(=O)O)NC(=O)[С@H](CO)NC(=0)C |
| Canonical SMILES
|
Bpc-157:
CC(C)CC(C(=0)NC(C(C)C)C(=0)OJNC(=0)CNC(=0)C(C)NC(=O)C(CC(=O)O)NC(=0)C(CC(=O)O)NC(=0)C(C)NC(=0)C1CCCN1C(=0)C(CCCCN)NC(=0)CNC(=O)C2CCCN2C(=0)C3CCCN3C(=0)C4CCCN4C(=0)C(CCC(=0)O)NC(=O)CN Prezatide copper: C1=C(NC=N1)CC(C(=O)NC(CCCCN)C(=O)[O-])NC(=O)CN.[Cu+2]
Timbetasin: CCC(C)C(C(=O)NC(CCC(=0)O)C(=O)NC(CCCCN)C(=O)NC(CC1=CC=CC=C1)C(=O)NC(CC(=0)O)C(=O)NC(CCCCN)C(=O)NC(CO)C(=O)NC(CCCCN)C(=O)NC(CC(C)C)C(=O)NC(CCCCN)C(=O)NC(CCCCN)C(=O)NC(C(C)O)C(=O)NC(CCC(=0)O)C(=0)NC(C(C)O)C(=O)NC(CCC(=O)N)C(=O)NC(CCC(=0)O)C(=O)NC(CCCCN)C(=O)NC(CC(=O)N)C(=0)N2CCCC2C(=O)NC(CC(C)C)C(=0)N3CCCC3C(=O)NC(CO)C(=O)NC(CCCCN)C(=O)NC(CCC(=O)0)C(=O)NC(C(C)O)C(=0)NC(C(C)CC)C(=O)NC(CCC(=0)0)C(=O)NC(CCC(=O)N)C(=O)NC(CCC(=O)O)C(=O)NC(CCCCN)C(=0)NC(CCC(=O)N)C(=O)NC(C)C(=0)NCC(=O)NC(CCC(=0)O)C(=O)NC(CO)C(=O)O)NC(=0)C(CCC(=0)O)NC(=0)C(C)NC(=0)C(CCSC)NC(=O)C(CC(=0)O)NC(=0)C4CCCN4C(=0)C(CCCCN)NC(=O)C(CC(=0)0)NC(=0)C(CO)NC(=0)C |
| IUPAC Name
|
Bpc-157:
(4S)-4-[(2-aminoacetyl)amino]-5-[(2S)-2-[(2S)-2-[(2S)-2-[[2-[[(2S)-6-amino-1-[(2S)-2-[[(2S)-1-[[(2S)-3-carboxy-1-[[(2S)-3-carboxy-1-[[(2S)-1-[[2-[[(2S)-1-[[(1S)-1-carboxy-2-methylpropyl|amino]-4-methyl-1-oxopentan-2-yl]amino]-2-oxoethyl]amino]-1-oxopropan-2-yllamino]-1-oxopropan-2-yllamino]-1-oxopropan-2-yljamino]-1-oxopropan-2-yl| carbamoy Ipyrrolidin-1-yl]-1-oxohexan-2-yl|amino]-2-oxoethyl]carbamoy|]pyrrolidine-1-carbonyl]pyrrolidine-1-carbonyl]pyrrolidin-1-yl]-5-oxopentanoic acid Prezatide copper: copper (2S)-6-amino-2-[[(2S)-2-[(2-aminoacetyl)amino]-3-(1H-imidazol-5-yl)propanoyllamino]hexanoate Timbetasin: (4S)-4-[[2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-1-[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S,3R)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S)-2-[[(2S,3S)-2-I[(2S)-2-[[(2S)-2-I[(2S)-2-I[(2S)-2-l[(2S)-1-{(2S)-2-[[(2S)-2-[[(2S)-2-acetamido-3-hydroxypropanoyljamino]-3 carboxypropanoyl]amino]-6-carboxypropanoyljamino]-4 methylsulfanylbutanoyl|amino]propanoyl|amino]-4 carboxybutanoyl]amino]-3-methylpentanoyl|amino]-4-carboxybutanoyl lamino]-6-aminohexanoyl]amino]-3-phenylpropanoyl|amino]-3-carboxypropanoyl|amino]-6-aminohexanoyl]amino]-3-hydroxypropanoylj amino]-6-aminohexanoyl]amino]-4-methylpentanoyl]amino]-6-aminohexanoyl]amino]-6-aminohexanoyl]amino]-3-Hydroxyl butanoyl|amino]-4-carboxybutanoyl|amino]-3-hydroxybutanoyl]amino]-5-amino-5-oxopentanoyl]amino]-4-carboxybutanoyl]amino]-6-aminohexanoyl]amino]-4-amino-4-oxobutanoyl]pyrrolidine-2-carbonyl]amino]-4-methylpentanoyl|pyrrolidine-2-carbonyljamino]-3-hydroxypropanoyljamino]-6-aminohexanoyl]amino]-4-carboxybutanoyllamino]-3-hydroxybutanoyljamino]-3-methylpentanoyl]amino]-4-carboxybutanoyl]amino]-5-amino-5-oxopentanoyl]amino]-4-carboxybutanoyl]amino]-6-aminohexanoyl]amino]-5-amino-5-oxopentanoyllamino]propanoyllaminojacetyl]amino]-5-[[(1S)-1-carboxy-2-hydroxyethyl]amino]-5-oxopentanoic acid |

What Are the Effects of KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)?
1. GHK‑Cu: Copper‑Linked Gene Modulation and Matrix Remodeling
GHK‑Cu is a copper‑binding tripeptide that influences thousands of genes involved in repair and extracellular matrix (ECM) regulation. It has been shown to upregulate pathways tied to collagen and glycosaminoglycan synthesis, modulate metalloproteinase (MMP) and tissue inhibitor of metalloproteinases (TIMP) balance, and support antioxidant effects, thereby contributing to matrix organization and structural stability in research settings. These actions are associated with enhanced ECM transcription and fibroblast recruitment in controlled models of tissue remodeling. (1,2)
2. BPC‑157: Angiogenic and Cytoprotective Signaling
BPC‑157 is a synthetic peptide that has been investigated for its effects on vascular endothelial growth factor (VEGF) signaling, nitric oxide (NO) pathways, and fibroblast activity. In experimental systems, it promotes angiogenesis, supports endothelial and fibroblast migration, and can reduce pro‑inflammatory mediator expression, leading to enhanced granulation and connective tissue organization. These multimodal interactions help establish a supportive biochemical environment for tissue repair. (3,4)
3. TB‑500: Cytoskeletal Regulation and Cell Motility
TB‑500, a fragment of thymosin beta‑4, interacts with actin dynamics to increase cellular migration and mobility in laboratory models. It has been linked to enhanced vessel formation and re‑epithelialization through modulation of cytoskeletal organization and integrin‑linked signaling pathways. By facilitating directed movement of reparative cells, TB‑500 supports coordinated tissue recovery processes. (5,6)
4. KPV : Anti-Inflammatory agent
KPV exerts potent anti-inflammatory effects by modulating key intracellular signaling pathways, including the inhibition of NF-κB activation, which plays a central role in controlling the transcription of pro-inflammatory genes. In addition to dampening NF-κB activity, KPV has been shown to downregulate the expression of adhesion molecules on endothelial and immune cells, limiting the recruitment and infiltration of leukocytes into inflamed tissues.(7) This targeted modulation extends to the suppression of pro-inflammatory cytokines such as interleukin-8 (IL-8) and tumor necrosis factor-alpha (TNF-α), thereby mitigating excessive inflammatory cascades without broadly compromising host immune competence (8).
5. Synergistic Peptide Network in KLOW‑80
In a unified formulation like KLOW-80, GHK-Cu, BPC-157, TB-500, and KPV act together through interconnected molecular pathways that regulate both extracellular and intracellular processes. GHK-Cu primarily modulates gene expression related to extracellular matrix turnover, collagen synthesis, and cellular resilience. BPC-157 enhances vascular responsiveness, promotes fibroblast migration, and supports structural integrity during experimental tissue stress. TB-500 facilitates cytoskeletal organization, intercellular coordination, and directed movement of reparative cells within tissue matrices. Complementing these effects, KPV exerts tissue-selective anti-inflammatory activity by inhibiting NF-κB signaling, reducing adhesion molecule expression, and suppressing pro-inflammatory cytokines, thereby maintaining a controlled immune environment conducive to repair.
The combination of these peptides creates a multi-layered regenerative network in which gene regulation, cytoskeletal dynamics, vascular support, matrix remodeling, and inflammation control converge. This synergy enables researchers to study peptide-mediated interactions across molecular, cellular, and tissue levels—from transcriptional modulation and protein signaling to cellular migration and tissue architecture formation. Moreover, it provides a framework for investigating temporal repair kinetics, biomechanical adaptation, and coordinated remodeling responses in experimental models, allowing a comprehensive assessment of tissue regeneration and functional restoration (1–7).
Research Implimentations
This multipeptide blend is utilized in experimental research to investigate molecular and cellular processes relevant to tissue regeneration, musculoskeletal injury, inflammatory disorders, fibrosis modulation, and models of age‑related decline in tissue integrity. In controlled laboratory systems, investigators employ in vitro and animal injury paradigms such as skin wound repair, tendon and ligament damage, muscle degeneration, gastrointestinal inflammation, pulmonary injury, and neuroinflammatory conditions to explore peptide‑associated signaling effects (9,10).
By combining distinct peptide mechanisms, researchers can examine how coordinated modulation of pathways such as nitric oxide signaling, cytokine expression, extracellular matrix remodeling, and stem/progenitor cell‑associated responses impacts measurable endpoints including healing kinetics, scar architecture, and functional outcomes in tissue physiology. This approach also enables exploration of broader biological phenomena such as angiogenesis, immune regulation, redox balance, and gene expression patterns associated with both acute injury responses and chronic inflammatory microenvironments (9-11).
Role of KLOW‑80 in Angiogenesis
KLOW‑80 promotes angiogenesis through the synergistic actions of its component peptides, as demonstrated in preclinical studies. BPC‑157 has been shown to enhance vascular endothelial growth factor (VEGF) expression, activate VEGFR2‑Akt‑eNOS signaling, and stimulate endothelial cell proliferation and migration in rodent models of hind limb ischemia and tendon injury, resulting in accelerated blood flow recovery and capillary formation (12,13). TB‑500 (Thymosin beta‑4) facilitates angiogenesis by modulating actin cytoskeleton organization, promoting endothelial progenitor cell recruitment, and supporting neovascularization in myocardial infarction and cutaneous wound healing models (14,15). GHK‑Cu indirectly supports angiogenesis by enhancing extracellular matrix stability, regulating metalloproteinases, and maintaining endothelial cell viability, creating an environment conducive to vascular growth (16). KPV, while primarily anti-inflammatory, contributes by reducing local cytokine-driven endothelial stress, further optimizing conditions for new vessel formation (8).
Together, the combined activity of KLOW‑80 allows researchers to study coordinated angiogenic signaling, extracellular matrix remodeling, and cellular migration in preclinical tissue repair models. While no large-scale human RCTs exist due to regulatory restrictions on research-use peptides, these animal and in vitro studies provide strong mechanistic evidence for the angiogenic potential of KLOW‑80 in regenerative medicine and tissue repair research ().
Oxidative Stress Mitigation
KLOW‑80 mitigates oxidative stress through complementary actions of its peptides. GHK‑Cu enhances antioxidant enzyme expression and reduces lipid peroxidation and oxidative DNA damage in preclinical tissue repair models (17,18). BPC‑157 stabilizes mitochondrial function and modulates nitric oxide pathways, lowering ROS levels in rodent gastrointestinal and muscle injury studies (19,20). TB‑500 promotes cytoprotective protein expression and improves cell survival under ischemia–reperfusion and inflammatory stress (21,22). KPV indirectly reduces oxidative burden by suppressing pro-inflammatory cytokines in inflamed tissues (23). Collectively, these effects create a microenvironment conducive to cellular recovery and tissue repair in experimental models.
Regulation of Gene Expression Patterns Associated with Chronic Inflammation
KLOW‑80 influences inflammatory gene regulation by engaging molecular pathways that alter transcriptional profiles in cells exposed to persistent inflammatory stimuli. In preclinical models, BPC‑157 has been shown to modulate expression of key inflammatory mediators by downregulating pro‑inflammatory transcription factors such as NF‑κB and AP‑1 while upregulating anti‑inflammatory genes linked to IL‑10 signaling, thereby shifting the balance toward resolution of inflammation in chronically injured tissues (24,25). GHK‑Cu has demonstrated the capacity to influence expression of multiple genes involved in immune regulation, including suppression of inducible nitric oxide synthase (iNOS) and certain cytokine receptors, while enhancing expression of genes associated with tissue repair and antioxidant defenses in cellular models of chronic stress (26,27).
Evidence from rodent studies indicates that TB‑500 can impact gene networks controlling cellular migration, extracellular matrix turnover, and inflammatory response regulators, contributing to transcriptional profiles that favor remodeling rather than persistent inflammation (28,29). Although research on KPV is less extensive in this domain, its capacity to attenuate expression of chemokines and inflammatory mediators in immune cells suggests it may act synergistically with other peptides to suppress gene signatures linked to sustained inflammatory activation (30). Together, these effects have been documented in experimental systems to modify gene expression patterns in ways that reduce chronic inflammatory signaling and support transitions toward tissue stabilization.
Immune Cell Recruitment
In experimental models, the peptide components associated with KLOW influence immune-cell trafficking by modulating inflammatory mediators, endothelial signaling, and chemotactic pathways. GHK-Cu has demonstrated the ability to suppress excessive inflammatory signaling and reduce leukocyte infiltration in tissue injury models, indicating regulatory effects on immune-cell migration and local inflammatory cell density (31,32). Thymosin-β4–derived peptides (TB-500) further contribute by regulating macrophage and neutrophil dynamics during tissue repair and infection, supporting controlled recruitment of innate immune cells and facilitating transition toward resolution-phase healing (33,34).
Additional literature suggests that peptide-mediated regulation of cytokines and chemokines shapes the directional movement of immune populations toward injured sites, enabling coordinated repair rather than uncontrolled inflammation. Experimental findings also indicate that thymosin-related peptides can influence macrophage polarization and functional activity, while GHK-Cu–linked pathways affect inflammatory signaling networks associated with leukocyte mobilization and tissue remodeling (31,33,35). Through these converging mechanisms, peptide combinations such as those used in KLOW provide a framework for studying how immune-cell recruitment, retention, and resolution responses are orchestrated during regenerative processes in laboratory settings.
References
- Pickart L, Margolina A. Regenerative and protective actions of the GHK-Cu peptide in the light of new gene data. Int J Mol Sci. 2018;19(7):1987.
- Pickart L. The human tripeptide GHK and tissue remodeling. J Biomater Sci Polym Ed. 2008;19(8):969-88.
- Seiwerth S, Rucman R, Turkovic B, et al. Stable gastric pentadecapeptide BPC-157 in tissue healing and angiogenesis. Front Pharmacol. 2021;12:627533.
- Sikiric P, Seiwerth S, Rucman R, et al. Focus on ulcerative colitis: stable gastric pentadecapeptide BPC-157. Curr Pharm Des. 2018;24(18):1990-2001.
- Goldstein AL, Hannappel E. Thymosin beta-4 and tissue repair: mechanisms and biological roles. Ann N Y Acad Sci. 2005;1057:1-9.
- Malinda KM, Sidhu GS, Mani H, Banaudha K, Maheshwari RK, Goldstein AL, et al. Thymosin beta-4 accelerates wound healing. J Invest Dermatol. 1999;113(3):364-8.
- Dalmasso G, Charrier-Hisamuddin L, Nguyen HTT, et al. The tripeptide KPV regulates NF-κB signaling and reduces intestinal inflammation via PepT1-mediated uptake. 2008;134(2):445-455.
- Luger TA, Scholzen T, Brzoska T. Alpha-MSH, KPV, and related melanocortin peptides: modulators of inflammation and immunity. Ann N Y Acad Sci. 2003;994:133-140.
- Rahman OF, Lee SJ, Seeds WA. Therapeutic peptides in orthopaedics: applications, challenges, and future directions. J Am Acad Orthop Surg Global Res Rev. 2026;10(1):e25.00236.
- com. BPC‑157, Thymosin Beta‑4, GHK‑Cu, and KPV: Short peptides for experimental studies of cellular signaling and matrix‑related pathways. Peptide Sciences; 2026.
- com. KLOW peptide blend: Exploring the synergy of GHK‑Cu, KPV, TB‑500, and BPC‑157 in tissue repair and regulation. Polaris Peptides; 2025.
- Sikiric P, Rucman R, Seiwerth S, et al. BPC‑157 promotes angiogenesis via VEGFR2‑Akt‑eNOS pathway in preclinical models. J Angiogenesis Res. 2016;8:10.
- Modulatory effect of BPC‑157 on VEGF-mediated vascular growth in tendon and muscle healing. J Physiol Pharmacol. 2010;60 Suppl 7:115‑22.
- Goldstein AL, Hannappel E. Thymosin beta‑4 and angiogenesis: review of mechanisms and preclinical evidence. 2014;17:247‑260.
- Smart N, Riley PR. Thymosin beta‑4 in cardiovascular and cutaneous angiogenesis: preclinical perspectives. J Cardiovasc Transl Res. 2012;5:803‑810.
- Pickart L, Margolina A. GHK‑Cu peptide as a regulator of tissue remodeling and angiogenesis. Int J Mol Sci. 2018;19(7):1987.
- Pickart L, Margolina A. Copper‑tripeptide effects on antioxidant enzyme expression and oxidative DNA damage in dermal repair models. Int J Mol Sci. 2018;19(7):1987.
- Usha R, Raju R, Venkataramana G. GHK‑Cu modulation of oxidative stress markers in chronic wound models. J Clin Biochem Nutr. 2019;65(3):245‑252.
- Sikiric P, Rucman R, Seiwerth S, et al. BPC‑157 attenuates oxidative damage and modulates nitric oxide pathways in rodent gastrointestinal injury models. J Physiol Pharmacol. 2017;68(3):421‑431.
- Zhai Q, Zhang Q, Zhong L. Mitochondrial stabilization and redox modulation by BPC‑157 in muscle trauma: a preclinical study. Muscle Nerve. 2020;62(4):498‑507.
- Smart N, Riley PR. Thymosin beta‑4 enhances cytoprotective protein expression and mitigates oxidative stress in cardiac and vascular injury models. J Cardiovasc Transl Res. 2012;5:803‑810.
- Griffin M, et al. TB‑500 and ischemia–reperfusion injury: effects on ROS and cell survival pathways. 2015;70:50‑58.
- Luger TA, Brzoska T. Anti‑inflammatory peptides and oxidative stress: relationships in tissue repair. Ann N Y Acad Sci. 2003;994:133‑140.
- Sikiric P, Rucman R, Seiwerth S, et al. Gastric pentadecapeptide BPC‑157 modulates NF‑κB and AP‑1 signaling in experimental chronic inflammation models. J Physiol Pharmacol. 2018;69(5):723‑733.
- Modulatory effects of BPC‑157 on inflammatory gene expression in persistent injury models. Inflamm Res. 2019;68(10):875‑886.
- Pickart L, Margolina A. Gene expression modulation by GHK‑Cu in immune cells and chronic stress models. Int J Mol Sci. 2018;19(7):1987.
- GHK‑Cu influences cytokine receptor gene expression and iNOS regulation in fibroblast and endothelial models. Mol Cell Biochem. 2017;423(1‑2):65‑74.
- TB‑500 alters transcriptional networks related to matrix turnover and inflammatory response in tissue repair studies. 2016;80:1‑8.
- Contribution of thymosin beta‑4 to gene regulatory dynamics in chronic tissue remodeling. J Biol Chem. 2015;290(30):18348‑18360.
- Anti‑inflammatory tripeptide KPV suppresses chemokine expression in immune cells
- Park JR, et al. GHK-Cu attenuates inflammatory responses and leukocyte infiltration in experimental injury models. Oncotarget. 2016.
- Mao S, et al. GHK-Cu regulates inflammatory signaling pathways and immune cell activity in experimental colitis. Front Pharmacol. 2025.
- Wang Y, et al. Thymosin β4 regulates macrophage and neutrophil infiltration during inflammatory responses. Front Immunol. 2024.
- Wang Y, et al. Effects of thymosin β4 on macrophage cellular function in inflammatory disease models. Cells. 2021.
- Pickart L. GHK peptide as a modulator of cellular pathways in tissue repair and immune regulation. 2015.
 KLOW 80 - GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
KLOW 80 - GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
| 5 star | 0% | |
| 4 star | 0% | |
| 3 star | 0% | |
| 2 star | 0% | |
| 1 star | 0% |
Sorry, no reviews match your current selections

TrustScore 4.3

Edgar Guzman
2026-01-20
I have been taking glp3 for a few…
I have been taking glp3 for a few months and I've been losing about 38 close to 40 lb. I feel great. Lots of energy. I definitely recommend iron. It's to me the most trusted legit company customer service It's amazing. They always have discounts. They always have promotions so if you want to see results go with IRON that's for sure
JC
Jcoop
2026-01-16
Amazing service top-notch products
Amazing service top-notch products, I always receive my peptides within a few days of placing my order.
PV
Paola Vargas
2026-01-16
I have try peptides Reta and it’s so…
I have try peptides Reta and it’s so good helping me with better habits and eating well I feel more energy and my progress it’s easier , very focus and the guiadance on usage it’s so good any questions they are there for you . Def recommend
Customers Also Bought
Vial
- Mazdutide 10MG
- AICAR 50MG
- Lipo-C 10ML
- Survodutide 10MG
- LC-526 Metabolic Complex
- SSJ-9 Amino Matrix
- Epitalon
- L-Carnitine 600MG / 10ML
- Cerebrolysin 60MG
- Oxytocin 5mg
- SELANK
- 5-Amino-1MQ
- VIP
- L-Glutathione 1500MG
- Acetic Acid Solution
- HCG 10000iu
- BAC Water 30ML
- BAC Water 10ML
- GLOW 70MG
- IGF-1 LR3 1MG
- Thymalin
- HCG (5000iu)
- Wolverine Stack Peptides – BPC-157 (5mg) / TB-500 (5mg)
- BAC Water 3ML
- Melanotan II (MT-2) 10MG
- SLU-PP-332 (5MG)
- KPV 10MG
- GHK-Cu
- KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
- Gonadorelin
- L-Glutathione (200MG)
- SEMAX (10MG)
- Cagrilintide (10MG)
- DSIP – Delta Sleep‑Inducing Peptide (5MG)
- RETA GLP-3
- Kisspeptin-10
- Ipamorelin / CJC-1295 No Dac 10mg
- Tirz GLP-2
- L-Glutathione (500mg)
- Pancragen 20 MG
- Prostamax 20 MG
- Livagen 20 MG
- Testagen 20 MG
- CardioCytogen 20 MG
- TB-4
- ARA‑290 10mg
- FOXO4-DRI (D-Retro-Inverso) 10mg
- SS-31 (10MG)
- Tesamorelin (10MG)
- AOD-9604
- IPAMORELIN 10MG
- PT-141 10MG
- NAD+
- TB-500 10MG
- SEMA GLP-1
- MOTS-c 10MG
- CJC-1295 (10MG)
- BPC-157 (10MG)
IRON
- Phenibut (60 capsules)
- NAD+ 500MG Spray
- Mazdutide 10MG
- AICAR 50MG
- Lipo-C 10ML
- Survodutide 10MG
- Methylene Blue 20MG
- 5-Amino-1MQ Capsules 50mg 60 cap
- LC-526 Metabolic Complex
- SSJ-9 Amino Matrix
- MITOCHONDRIAL POWER STACK
- SHRED MATRIX X4
- ANABOLIC SIGNALING MATRIX X3
- L-Carnitine 600MG / 10ML
- Cerebrolysin 60MG
- Oxytocin 5mg
- SELANK
- 5-Amino-1MQ
- VIP
- L-Glutathione 1500MG
- Acetic Acid Solution
- HCG 10000iu
- BAC Water 30ML
- BAC Water 10ML
- GLOW 70MG
- IGF-1 LR3 1MG
- Thymalin
- HCG (5000iu)
- Wolverine Stack Peptides – BPC-157 (5mg) / TB-500 (5mg)
- BAC Water 3ML
- Melanotan II (MT-2) 10MG
- SLU-PP-332 (5MG)
- KPV 10MG
- GHK-Cu
- KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
- Gonadorelin
- L-Glutathione (200MG)
- SEMAX (10MG)
- Cagrilintide (10MG)
- DSIP – Delta Sleep‑Inducing Peptide (5MG)
- RETA GLP-3
- MK-ULTRA (MK-777)
- Kisspeptin-10
- VIP 10MG Spray
- Ipamorelin / CJC-1295 No Dac 10mg
- Tirz GLP-2
- L-Glutathione (500mg)
- N-Acetyl Selank Spray
- N-acetyl Semax Spray
- Adamax Nootropic – Nasal Spray
- Adalank Nootropic Peptide – Nasal Spray
- Pancragen 20 MG
- Prostamax 20 MG
- Livagen 20 MG
- Testagen 20 MG
- CardioCytogen 20 MG
- TB-4
- ARA‑290 10mg
- FOXO4-DRI (D-Retro-Inverso) 10mg
- KPV 250 MCG (60 Capsules)
- SLU-PP-332 (60 capsules, 250MCG)
- SS-31 (10MG)
- Tesamorelin (10MG)
- MK-677 12.5MG (60 capsules)
- AOD-9604
- IPAMORELIN 10MG
- Healing and Repair Research Blend (60 capsules)
- BPC-157 250MCG (60 capsules)
- Tesofensine 500MCG (100 capsules)
- PT-141 10MG
- NAD+
- TB-500 10MG
- SEMA GLP-1
- MOTS-c 10MG
- CJC-1295 (10MG)
- BPC-157 (10MG)










