Ipamorelin / CJC-1295 No Dac 10mg
$129.99
Shipping Calculated at checkout.
Availability: 188 in stock
FREE SHIPPING
99%+ PURITY
MADE IN USA
Vial
- Mazdutide 10MG
- AICAR 50MG
- Lipo-C 10ML
- Survodutide 10MG
- LC-526 Metabolic Complex
- SSJ-9 Amino Matrix
- Epitalon
- L-Carnitine 600MG / 10ML
- Cerebrolysin 60MG
- Oxytocin 5mg
- SELANK
- 5-Amino-1MQ
- VIP
- L-Glutathione 1500MG
- Acetic Acid Solution
- HCG 10000iu
- BAC Water 30ML
- BAC Water 10ML
- GLOW 70MG
- IGF-1 LR3 1MG
- Thymalin
- HCG (5000iu)
- Wolverine Stack Peptides – BPC-157 (5mg) / TB-500 (5mg)
- BAC Water 3ML
- Melanotan II (MT-2) 10MG
- SLU-PP-332 (5MG)
- KPV 10MG
- GHK-Cu
- KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
- Gonadorelin
- L-Glutathione (200MG)
- SEMAX (10MG)
- Cagrilintide (10MG)
- DSIP – Delta Sleep‑Inducing Peptide (5MG)
- RETA GLP-3
- Kisspeptin-10
- Ipamorelin / CJC-1295 No Dac 10mg
- Tirz GLP-2
- L-Glutathione (500mg)
- Pancragen 20 MG
- Prostamax 20 MG
- Livagen 20 MG
- Testagen 20 MG
- CardioCytogen 20 MG
- TB-4
- ARA‑290 10mg
- FOXO4-DRI (D-Retro-Inverso) 10mg
- SS-31 (10MG)
- Tesamorelin (10MG)
- AOD-9604
- IPAMORELIN 10MG
- PT-141 10MG
- NAD+
- TB-500 10MG
- SEMA GLP-1
- MOTS-c 10MG
- CJC-1295 (10MG)
- BPC-157 (10MG)
IRON
- Phenibut (60 capsules)
- NAD+ 500MG Spray
- Mazdutide 10MG
- AICAR 50MG
- Lipo-C 10ML
- Survodutide 10MG
- Methylene Blue 20MG
- 5-Amino-1MQ Capsules 50mg 60 cap
- LC-526 Metabolic Complex
- SSJ-9 Amino Matrix
- MITOCHONDRIAL POWER STACK
- SHRED MATRIX X4
- ANABOLIC SIGNALING MATRIX X3
- L-Carnitine 600MG / 10ML
- Cerebrolysin 60MG
- Oxytocin 5mg
- SELANK
- 5-Amino-1MQ
- VIP
- L-Glutathione 1500MG
- Acetic Acid Solution
- HCG 10000iu
- BAC Water 30ML
- BAC Water 10ML
- GLOW 70MG
- IGF-1 LR3 1MG
- Thymalin
- HCG (5000iu)
- Wolverine Stack Peptides – BPC-157 (5mg) / TB-500 (5mg)
- BAC Water 3ML
- Melanotan II (MT-2) 10MG
- SLU-PP-332 (5MG)
- KPV 10MG
- GHK-Cu
- KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
- Gonadorelin
- L-Glutathione (200MG)
- SEMAX (10MG)
- Cagrilintide (10MG)
- DSIP – Delta Sleep‑Inducing Peptide (5MG)
- RETA GLP-3
- MK-ULTRA (MK-777)
- Kisspeptin-10
- VIP 10MG Spray
- Ipamorelin / CJC-1295 No Dac 10mg
- Tirz GLP-2
- L-Glutathione (500mg)
- N-Acetyl Selank Spray
- N-acetyl Semax Spray
- Adamax Nootropic – Nasal Spray
- Adalank Nootropic Peptide – Nasal Spray
- Pancragen 20 MG
- Prostamax 20 MG
- Livagen 20 MG
- Testagen 20 MG
- CardioCytogen 20 MG
- TB-4
- ARA‑290 10mg
- FOXO4-DRI (D-Retro-Inverso) 10mg
- KPV 250 MCG (60 Capsules)
- SLU-PP-332 (60 capsules, 250MCG)
- SS-31 (10MG)
- Tesamorelin (10MG)
- MK-677 12.5MG (60 capsules)
- AOD-9604
- IPAMORELIN 10MG
- Healing and Repair Research Blend (60 capsules)
- BPC-157 250MCG (60 capsules)
- Tesofensine 500MCG (100 capsules)
- PT-141 10MG
- NAD+
- TB-500 10MG
- SEMA GLP-1
- MOTS-c 10MG
- CJC-1295 (10MG)
- BPC-157 (10MG)

What is Ipamorelin / CJC-1295 No Dac 10mg?
Introduction
Peptide-based regulators of growth hormone (GH) secretion have long been employed as investigative tools to elucidate endocrine signaling pathways, feedback mechanisms, and the physiological basis of pulsatile hormone release (7,8). Among these, combinations of structurally and functionally distinct growth hormone secretagogues are of particular interest in experimental endocrinology, as they enable concurrent examination of parallel receptor systems involved in pituitary regulation (1). The research blend of CJC-1295 (without DAC) and Ipamorelin represents one such model and is frequently utilized to study coordinated modulation of the growth hormone axis under controlled laboratory conditions (9). CJC-1295 (Mod GRF 1–29) is a synthetic analogue derived from the biologically active region of endogenous growth hormone–releasing hormone. Structural modifications confer enhanced resistance to enzymatic degradation while preserving receptor specificity, enabling reproducible stimulation of pituitary somatotrophs via GHRH receptor engagement (3,4). This interaction primarily activates adenylate cyclase dependent signaling cascades, providing a reliable framework for examining cAMP-mediated regulation of GH synthesis and secretion (10). Ipamorelin, in contrast, belongs to the class of selective ghrelin receptor agonists and induces growth hormone release through an alternative intracellular mechanism involving phospholipase C activation and calcium mobilization (5,6). Its high receptor selectivity makes it particularly useful for isolating ghrelin-mediated signaling with minimal engagement of non-target pituitary pathways (11).When investigated together, CJC-1295 (no DAC) and Ipamorelin provide a complementary signaling model that reflects the multi-pathway control of GH pulsatility observed in physiological systems (12). This dual-peptide approach has been applied in preclinical and in vitro studies to explore signal integration, temporal hormone release dynamics, and regulatory interactions within the hypothalamic pituitary axis (13). As such, the CJC-1295 and Ipamorelin blend continues to serve as a valuable experimental construct for advancing mechanistic understanding of peptide-driven endocrine regulation rather than for clinical or therapeutic interpretation.

Chemical Structure of Ipamorelin / CJC-1295 No Dac 10mg
Ipamorelin
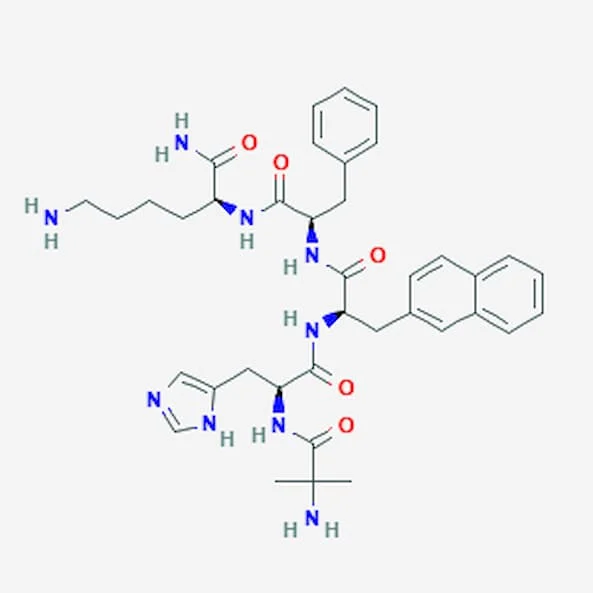
Source: PubChem
-
Sequence: Aib-His-D-2-Nal-D-Phe-Lys-NH₂
-
CAS Number: 170851-70-4
-
Molecular Formula: C₃₈H₄₉N₉O₅
-
Peptide Type: Pentapeptide, ghrelin mimetic
CJC-1295 Without DAC (Mod GRF 1-29)
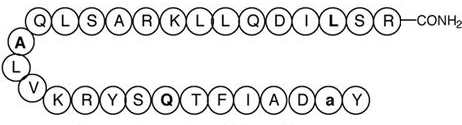
Source: Oxford Academic
-
Sequence: Tyr-Ala-Asp-Ala-Phe-Gln-Ser-Tyr-Arg-Lys-Val-Leu-Ala-Gln-Leu-Ser-Ala-Arg-Lys-Leu-Leu-Gln-Asp-Leu-Ser-Arg-NH₂
-
CAS Number: 446262-90-4
-
Molecular Formula: C₁₅₂H₂₅₂N₄₄O₄₂
-
Peptide Type: GHRH analog, growth hormone secretagogue

What Are the Effects of Ipamorelin / CJC-1295 No Dac 10mg?
CJC-1295 (No DAC) is a synthetic peptide based on the receptor-active segment of endogenous growth hormone–releasing hormone, encompassing its initial 29 amino acids (4,14). Structural modifications were introduced to enhance stability against enzymatic degradation while maintaining strong specificity for the GHRH receptor expressed on pituitary somatotroph cells (3). This selective receptor interaction enables reproducible activation of intracellular signaling pathways associated with growth hormone synthesis and secretion (10). Owing to its increased resistance to proteolysis and consistent receptor engagement, CJC-1295 (No DAC) is commonly utilized in experimental models to investigate GHRH mediated signaling and regulatory mechanisms governing growth hormone release (15).
Ipamorelin is a short-chain, synthetically produced peptide composed of five amino acids that functions as a targeted activator of the growth hormone secretagogue receptor subtype 1a (GHSR-1a) (1,6). It was specifically designed to replicate ghrelin-associated growth hormone signaling while maintaining a narrow receptor interaction profile (11). Unlike earlier secretagogues with broader endocrine activity, Ipamorelin demonstrates a high degree of selectivity for GHSR-1a, allowing researchers to examine ghrelin-mediated intracellular pathways with reduced interference from parallel pituitary hormone systems (5). Engagement of this receptor primarily initiates phospholipase C dependent signaling and intracellular calcium mobilization within somatotroph cells, supporting its utility in mechanistic investigations of growth hormone release dynamics (7,13). Due to its receptor specificity and predictable signaling behavior, Ipamorelin is frequently incorporated into in vitro and preclinical models aimed at studying hypothalamic pituitary communication, signal transduction fidelity, and temporal hormone secretion patterns under controlled experimental conditions (11,15).
Mechanism of action
CJC-1295 (No DAC) engages the growth hormone, releasing hormone receptor (GHRHR), leading to activation of Gₛ protein coupled signaling. This stimulates adenylate cyclase activity, resulting in elevated intracellular cyclic AMP levels and downstream activation of protein kinase A, which supports growth hormone related cellular responses.
Ipamorelin selectively binds to the growth hormone secretagogue receptor type 1a (GHSR-1a), triggering Gq/11-dependent signaling pathways. This interaction activates phospholipase C, promotes inositol triphosphate formation, and induces the release of calcium from intracellular stores, contributing to growth hormone secretory processes.
Concurrent evaluation of both peptides allows researchers to examine how cAMP-driven and calcium-dependent signaling pathways operate simultaneously within pituitary somatotrophs, providing a controlled model for studying pathway integration, receptor cross-communication, and endocrine signal coordination. Here is the representataion of its mechanistic pathway in form of flowchart and diagram.
Research Applications of the CJC-1295 and Ipamorelin Blend
- Dual-receptor signaling analysis (GHRHR and GHSR-1a)
The combined use of CJC-1295 and Ipamorelin enables simultaneous activation of growth hormone–releasing hormone receptors (GHRHR) and ghrelin receptors (GHSR-1a), providing an experimental model to study parallel receptor engagement and convergence at the level of pituitary somatotroph signaling. (14,16) - Pulsatile endocrine signaling and receptor cross-talk studies
By engaging mechanistically distinct receptor systems, this peptide pairing supports investigation of growth hormone pulsatility and temporal coordination of endocrine signals. Preclinical studies have shown that co-activation of GHRHR and GHSR-1a offers insight into receptor cross-talk and integrated hypothalamic–pituitary regulation(17,18) - Evaluation of cAMP- and Ca²⁺-dependent second messenger pathways
CJC-1295 primarily stimulates Gₛ-coupled signaling pathways leading to increased intracellular cAMP, whereas Ipamorelin activates Gq/11-linked signaling and calcium mobilization. Their combined application allows comparative assessment of second messenger dynamics and intracellular signal integration in endocrine research systems. (19,20). - Downstream IGF-axis modulation in preclinical models
In experimental settings, upstream modulation of growth hormone secretion using CJC-1295 and Ipamorelin has been employed to explore mechanistic relationships between pituitary signaling and downstream components of the insulin-like growth factor axis, focusing on pathway regulation rather than therapeutic interpretation. (21,22)
Preclinical Research Summary
Experimental investigations conducted in controlled laboratory settings have extensively explored growth hormone regulating peptides, including growth hormone releasing hormone analogues and ghrelin receptor agonists, to elucidate fundamental mechanisms of endocrine regulation. Using in vitro systems and animal based models, these studies have focused on characterizing receptor specificity, intracellular signaling fidelity, and the temporal organization of hormone secretion.
Within these preclinical frameworks, particular attention has been given to how distinct receptor pathways contribute to pulsatile growth hormone release and how parallel signaling inputs are integrated at the level of pituitary somatotrophs. Cellular assays have enabled detailed examination of second messenger dynamics, such as cyclic AMP generation and calcium mobilization, while animal models have provided insight into systemic signal coordination and feedback regulation along the hypothalamic pituitary axis.
Importantly, all observations derived from this body of research originate from non-human and non-clinical experimental environments. The findings are intended to advance mechanistic understanding of endocrine signaling architecture and regulatory complexity, rather than to inform therapeutic use or clinical application.
References
- Smith RG, Van der Ploeg LH, Howard AD, et al. Peptidomimetic regulation of growth hormone secretion. Endocr Rev. 1997;18(5):621–645.
- Thorner MO, Vance ML, Laws ER, Horvath E, Kovacs K. The anterior pituitary. In: Williams Textbook of Endocrinology. 10th ed. Philadelphia: Saunders; 2003. p. 249–340.
- Momany FA, Bowers CY, Reynolds GA, et al. Design, synthesis, and biological activity of growth hormone-releasing hormone analogs. Endocrinology. 1984;114(5):1531–1536.
- Frohman LA, Jansson JO. Growth hormone-releasing hormone. Endocr Rev. 1986;7(3):223–253.
- Raun K, Hansen BS, Johansen NL, et al. Ipamorelin, a selective growth hormone secretagogue with minimal endocrine side effects. J Endocrinol. 2001;170(2):435–443.
- Howard AD, Feighner SD, Cully DF, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273(5277):974–977.
- Muller EE, Locatelli V, Cocchi D. Neuroendocrine control of growth hormone secretion. Physiol Rev. 1999;79(2):511–607.
- Thorner MO, et al. Growth hormone–releasing hormone and regulation of growth hormone secretion. Endocr Rev. 1996;17(6):671–728.
- Vance ML, Kaiser DL, Frohman LA, Rivier J, Vale W. Dual secretagogue regulation of growth hormone release. J Clin Endocrinol Metab. 1985;60(3):522–528.
- Mayo KE, Miller T, DeAlmeida V, et al. Regulation of growth hormone gene expression by GHRH. Recent Prog Horm Res. 2000;55:257–283.
- Bednarek MA, et al. Structure–function studies of ghrelin receptor agonists. J Med Chem. 2000;43(23):4370–4376.
- Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion. J Endocrinol Invest. 1998;21(9):585–594.
- Kojima M, Kangawa K. Ghrelin: structure and biological actions. Physiol Rev. 2005;85(2):495–522.
- Thorner MO, et al. Growth hormone–releasing hormone and the regulation of growth hormone secretion. Endocr Rev. 1996;17(6):671–728.
- Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion. J Endocrinol Invest. 1998;21(9):585–594.
- Mayo KE, Miller LJ, Bataille D, Dalle S, Göke B, Thorens B, et al. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol Rev. 2003;55(1):167-194.
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656-660.
- Smith RG, Sun Y, Betancourt L, Asnicar M. Growth hormone secretagogues: receptor specificity and signal transduction. Endocrine. 2005;28(1):3-14.
- Gaylinn BD. Regulation of growth hormone secretion by GHRH and ghrelin signaling pathways. Endocrinol Metab Clin North Am. 2017;46(2):211-224.
- van der Lely AJ, Tschöp M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological and pharmacological aspects of ghrelin. Endocr Rev. 2004;25(3):426-457.
- Le Roith D, Bondy C, Yakar S, Liu JL, Butler A. The somatomedin hypothesis: 2001. Endocr Rev. 2001;22(1):53-74.
- Brooks AJ, Waters MJ. The growth hormone receptor: mechanism of activation and implications for signal transduction. Nat Rev Endocrinol. 2010;6(9):515-525.
 Ipamorelin / CJC-1295 No Dac 10mg
Ipamorelin / CJC-1295 No Dac 10mg
| 5 star | 0% | |
| 4 star | 0% | |
| 3 star | 0% | |
| 2 star | 0% | |
| 1 star | 0% |
Sorry, no reviews match your current selections

TrustScore 4.3

Edgar Guzman
2026-01-20
I have been taking glp3 for a few…
I have been taking glp3 for a few months and I've been losing about 38 close to 40 lb. I feel great. Lots of energy. I definitely recommend iron. It's to me the most trusted legit company customer service It's amazing. They always have discounts. They always have promotions so if you want to see results go with IRON that's for sure
JC
Jcoop
2026-01-16
Amazing service top-notch products
Amazing service top-notch products, I always receive my peptides within a few days of placing my order.
PV
Paola Vargas
2026-01-16
I have try peptides Reta and it’s so…
I have try peptides Reta and it’s so good helping me with better habits and eating well I feel more energy and my progress it’s easier , very focus and the guiadance on usage it’s so good any questions they are there for you . Def recommend
Vial
- Mazdutide 10MG
- AICAR 50MG
- Lipo-C 10ML
- Survodutide 10MG
- LC-526 Metabolic Complex
- SSJ-9 Amino Matrix
- Epitalon
- L-Carnitine 600MG / 10ML
- Cerebrolysin 60MG
- Oxytocin 5mg
- SELANK
- 5-Amino-1MQ
- VIP
- L-Glutathione 1500MG
- Acetic Acid Solution
- HCG 10000iu
- BAC Water 30ML
- BAC Water 10ML
- GLOW 70MG
- IGF-1 LR3 1MG
- Thymalin
- HCG (5000iu)
- Wolverine Stack Peptides – BPC-157 (5mg) / TB-500 (5mg)
- BAC Water 3ML
- Melanotan II (MT-2) 10MG
- SLU-PP-332 (5MG)
- KPV 10MG
- GHK-Cu
- KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
- Gonadorelin
- L-Glutathione (200MG)
- SEMAX (10MG)
- Cagrilintide (10MG)
- DSIP – Delta Sleep‑Inducing Peptide (5MG)
- RETA GLP-3
- Kisspeptin-10
- Ipamorelin / CJC-1295 No Dac 10mg
- Tirz GLP-2
- L-Glutathione (500mg)
- Pancragen 20 MG
- Prostamax 20 MG
- Livagen 20 MG
- Testagen 20 MG
- CardioCytogen 20 MG
- TB-4
- ARA‑290 10mg
- FOXO4-DRI (D-Retro-Inverso) 10mg
- SS-31 (10MG)
- Tesamorelin (10MG)
- AOD-9604
- IPAMORELIN 10MG
- PT-141 10MG
- NAD+
- TB-500 10MG
- SEMA GLP-1
- MOTS-c 10MG
- CJC-1295 (10MG)
- BPC-157 (10MG)
IRON
- Phenibut (60 capsules)
- NAD+ 500MG Spray
- Mazdutide 10MG
- AICAR 50MG
- Lipo-C 10ML
- Survodutide 10MG
- Methylene Blue 20MG
- 5-Amino-1MQ Capsules 50mg 60 cap
- LC-526 Metabolic Complex
- SSJ-9 Amino Matrix
- MITOCHONDRIAL POWER STACK
- SHRED MATRIX X4
- ANABOLIC SIGNALING MATRIX X3
- L-Carnitine 600MG / 10ML
- Cerebrolysin 60MG
- Oxytocin 5mg
- SELANK
- 5-Amino-1MQ
- VIP
- L-Glutathione 1500MG
- Acetic Acid Solution
- HCG 10000iu
- BAC Water 30ML
- BAC Water 10ML
- GLOW 70MG
- IGF-1 LR3 1MG
- Thymalin
- HCG (5000iu)
- Wolverine Stack Peptides – BPC-157 (5mg) / TB-500 (5mg)
- BAC Water 3ML
- Melanotan II (MT-2) 10MG
- SLU-PP-332 (5MG)
- KPV 10MG
- GHK-Cu
- KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
- Gonadorelin
- L-Glutathione (200MG)
- SEMAX (10MG)
- Cagrilintide (10MG)
- DSIP – Delta Sleep‑Inducing Peptide (5MG)
- RETA GLP-3
- MK-ULTRA (MK-777)
- Kisspeptin-10
- VIP 10MG Spray
- Ipamorelin / CJC-1295 No Dac 10mg
- Tirz GLP-2
- L-Glutathione (500mg)
- N-Acetyl Selank Spray
- N-acetyl Semax Spray
- Adamax Nootropic – Nasal Spray
- Adalank Nootropic Peptide – Nasal Spray
- Pancragen 20 MG
- Prostamax 20 MG
- Livagen 20 MG
- Testagen 20 MG
- CardioCytogen 20 MG
- TB-4
- ARA‑290 10mg
- FOXO4-DRI (D-Retro-Inverso) 10mg
- KPV 250 MCG (60 Capsules)
- SLU-PP-332 (60 capsules, 250MCG)
- SS-31 (10MG)
- Tesamorelin (10MG)
- MK-677 12.5MG (60 capsules)
- AOD-9604
- IPAMORELIN 10MG
- Healing and Repair Research Blend (60 capsules)
- BPC-157 250MCG (60 capsules)
- Tesofensine 500MCG (100 capsules)
- PT-141 10MG
- NAD+
- TB-500 10MG
- SEMA GLP-1
- MOTS-c 10MG
- CJC-1295 (10MG)
- BPC-157 (10MG)