BPC-157 250MCG (60 capsules)
$109.99
Shipping Calculated at checkout.
Availability: 180 in stock
FREE SHIPPING
99%+ PURITY
MADE IN USA
Vial
- Mazdutide 10MG
- AICAR 50MG
- Lipo-C 10ML
- Survodutide 10MG
- LC-526 Metabolic Complex
- SSJ-9 Amino Matrix
- Epitalon
- L-Carnitine 600MG / 10ML
- Cerebrolysin 60MG
- Oxytocin 5mg
- SELANK
- 5-Amino-1MQ
- VIP
- L-Glutathione 1500MG
- Acetic Acid Solution
- HCG 10000iu
- BAC Water 30ML
- BAC Water 10ML
- GLOW 70MG
- IGF-1 LR3 1MG
- Thymalin
- HCG (5000iu)
- Wolverine Stack Peptides – BPC-157 (5mg) / TB-500 (5mg)
- BAC Water 3ML
- Melanotan II (MT-2) 10MG
- SLU-PP-332 (5MG)
- KPV 10MG
- GHK-Cu
- KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
- Gonadorelin
- L-Glutathione (200MG)
- SEMAX (10MG)
- Cagrilintide (10MG)
- DSIP – Delta Sleep‑Inducing Peptide (5MG)
- RETA GLP-3
- Kisspeptin-10
- Ipamorelin / CJC-1295 No Dac 10mg
- Tirz GLP-2
- L-Glutathione (500mg)
- Pancragen 20 MG
- Prostamax 20 MG
- Livagen 20 MG
- Testagen 20 MG
- CardioCytogen 20 MG
- TB-4
- ARA‑290 10mg
- FOXO4-DRI (D-Retro-Inverso) 10mg
- SS-31 (10MG)
- Tesamorelin (10MG)
- AOD-9604
- IPAMORELIN 10MG
- PT-141 10MG
- NAD+
- TB-500 10MG
- SEMA GLP-1
- MOTS-c 10MG
- CJC-1295 (10MG)
- BPC-157 (10MG)
IRON
- Phenibut (60 capsules)
- NAD+ 500MG Spray
- Mazdutide 10MG
- AICAR 50MG
- Lipo-C 10ML
- Survodutide 10MG
- Methylene Blue 20MG
- 5-Amino-1MQ Capsules 50mg 60 cap
- LC-526 Metabolic Complex
- SSJ-9 Amino Matrix
- MITOCHONDRIAL POWER STACK
- SHRED MATRIX X4
- ANABOLIC SIGNALING MATRIX X3
- L-Carnitine 600MG / 10ML
- Cerebrolysin 60MG
- Oxytocin 5mg
- SELANK
- 5-Amino-1MQ
- VIP
- L-Glutathione 1500MG
- Acetic Acid Solution
- HCG 10000iu
- BAC Water 30ML
- BAC Water 10ML
- GLOW 70MG
- IGF-1 LR3 1MG
- Thymalin
- HCG (5000iu)
- Wolverine Stack Peptides – BPC-157 (5mg) / TB-500 (5mg)
- BAC Water 3ML
- Melanotan II (MT-2) 10MG
- SLU-PP-332 (5MG)
- KPV 10MG
- GHK-Cu
- KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
- Gonadorelin
- L-Glutathione (200MG)
- SEMAX (10MG)
- Cagrilintide (10MG)
- DSIP – Delta Sleep‑Inducing Peptide (5MG)
- RETA GLP-3
- MK-ULTRA (MK-777)
- Kisspeptin-10
- VIP 10MG Spray
- Ipamorelin / CJC-1295 No Dac 10mg
- Tirz GLP-2
- L-Glutathione (500mg)
- N-Acetyl Selank Spray
- N-acetyl Semax Spray
- Adamax Nootropic – Nasal Spray
- Adalank Nootropic Peptide – Nasal Spray
- Pancragen 20 MG
- Prostamax 20 MG
- Livagen 20 MG
- Testagen 20 MG
- CardioCytogen 20 MG
- TB-4
- ARA‑290 10mg
- FOXO4-DRI (D-Retro-Inverso) 10mg
- KPV 250 MCG (60 Capsules)
- SLU-PP-332 (60 capsules, 250MCG)
- SS-31 (10MG)
- Tesamorelin (10MG)
- MK-677 12.5MG (60 capsules)
- AOD-9604
- IPAMORELIN 10MG
- Healing and Repair Research Blend (60 capsules)
- BPC-157 250MCG (60 capsules)
- Tesofensine 500MCG (100 capsules)
- PT-141 10MG
- NAD+
- TB-500 10MG
- SEMA GLP-1
- MOTS-c 10MG
- CJC-1295 (10MG)
- BPC-157 (10MG)

What is BPC-157 250MCG (60 capsules)?
Introduction
Experimental studies have explored the Involvement of BPC-157 in biological processes related to tissue repair, inflammatory modulation, and wound recovery across multiple body systems. Research models indicate potential effects on musculoskeletal structures such as muscles, tendons, and ligaments, as well as on neural and gastrointestinal tissues. A frequently investigated mechanism is its association with angiogenesis, a process critical for restoring blood supply to damaged tissues by facilitating the formation of new blood vessels. Adequate vascularization supports oxygen and nutrient delivery, which is essential for tissue recovery. In gastrointestinal research models it has been examined for its interaction with mucosal integrity, including its potential to reduce damage associated with nonsteroidal anti-inflammatory drug exposure and to support gastric barrier function. Neurological research has further examined its involvement in neuroprotective pathways. Animal-based studies suggest potential effects on nerve repair mechanisms and recovery processes following experimental models of traumatic brain and spinal cord injury. Additional investigations have assessed its influence on neurotransmitter regulation, indicating possible relevance to neural signaling balance.Current findings are derived primarily from preclinical and experimental research settings. Further controlled human studies are necessary to clarify safety parameters, long term outcomes, and any potential translational relevance within clinical research frameworks.

Chemical Structure of BPC-157 250MCG (60 capsules)
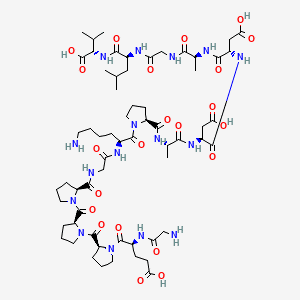
image source : Pubchem
Sequence: Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val
Molecular Formula: C₆₂H₉₈N₁₆O₂₂
Molecular Weight: 1419.556 g/mol
PubChem CID: 108101

What Are the Effects of BPC-157 250MCG (60 capsules)?
- Regulation of Vascular Repair Pathways
BPC-157 has been shown in experimental models to influence vascular repair by supporting endothelial function and microcirculatory stability. Its activity is associated with signaling networks involved in blood vessel formation and maintenance, which are essential during the early stages of tissue recovery. Improved vascular responsiveness facilitates efficient nutrient and oxygen delivery to compromised regions, creating a favorable environment for regeneration.
- Modulation of Inflammatory Responses
Another proposed mechanism involves the regulation of inflammatory cascades. it appears to attenuate excessive inflammatory signaling by influencing cytokine activity and cellular stress responses. By limiting prolonged inflammation, it may reduce secondary tissue damage and support a controlled healing environment.
- Support of Cellular Protection and Survival
Research suggests that it also contributes to cytoprotective processes by enhancing cellular resistance to chemical, mechanical, or ischemic stress. This protective effect is linked to stabilization of cell membranes and preservation of intracellular homeostasis, allowing cells to maintain function during injury-related stress.
- Influence on Connective Tissue Remodeling
In musculoskeletal research models, it has been associated with altered fibroblast behavior and extracellular matrix organization. These effects may support orderly collagen deposition and structural realignment during the repair of tendons, ligaments, and muscle tissue, contributing to improved biomechanical recovery.
- Maintenance of Gastrointestinal Barrier Integrity
Within the gastrointestinal system, it has been investigated for its role in preserving epithelial continuity and local perfusion. Experimental findings indicate enhanced resistance of the mucosal lining to chemical or drug-induced injury, suggesting involvement in barrier maintenance and localized repair mechanisms.
- Interaction with Neural Repair Processes
Neuroscientific studies have explored BPC-157’s involvement in neural recovery pathways. Evidence from animal models points toward support of axonal continuity, synaptic stability, and functional recovery following nervous system injury. These effects appear to arise indirectly through vascular support, inflammation control, and cellular protection rather than direct neurotransmitter stimulation.
- Integrated Multi-Pathway Activity
Overall, BPC-157 is thought to act through a coordinated network of biological pathways rather than a single receptor-mediated mechanism. Its experimental effects reflect combined actions on vascular regulation, inflammatory balance, cellular protection, and structural remodeling, collectively supporting tissue adaptation and recovery under stress conditions.
BPC-157 is a laboratory-synthesized peptide modeled from a protective compound originally identified in gastric secretions. It has attracted research interest due to its observed biological activity in experimental models related to tissue recovery and cellular protection. Preclinical studies have explored its involvement in repair processes, vascular adaptation, and inflammatory regulation, positioning it as a molecule of interest in musculoskeletal and gastrointestinal research contexts (1).
Evidence from experimental investigations suggests that BPC-157 supports recovery in connective, muscular, and neural tissues through interactions with molecular signaling systems associated with growth regulation and nitric oxide balance. These mechanisms have been linked to improved structural restoration and functional recovery in models of soft tissue damage. Additional findings indicate potential benefits in experimental gastrointestinal injury models, including attenuation of inflammatory responses and enhanced mucosal resilience (2).
Neuroscientific research has further examined the peptide’s interaction with monoaminergic pathways, particularly those involving dopamine and serotonin signaling. Such interactions have been associated with protective effects in nervous system injury models and may contribute to functional stabilization following neurological stress (3).
Animal-based studies have also reported that BPC-157 may mitigate drug-induced tissue stress, enhance microcirculatory dynamics, and reduce markers of oxidative damage. Its influence on intracellular repair signaling networks has led to growing research interest within experimental sports science and orthopedic models focused on tissue adaptation and recovery (4,5).
Despite these promising findings, current evidence remains largely preclinical. Rigorous human studies are required to determine safety parameters, mechanistic specificity, and potential translational relevance before any clinical applications can be considered.
1. Tissue Healing and Regenerative Activity
BPC-157 has been extensively examined in experimental research for its involvement in biological repair processes affecting a wide range of tissues. Since the mid-2010s, multiple studies have explored its effects on musculoskeletal structures, including skeletal muscle, connective tissue, and bone, highlighting its relevance in regenerative research models.
Skeletal Muscle Recovery
Experimental findings suggest that it supports muscle repair by improving local vascular responses at sites of injury. This effect has been linked to enhanced microvascular development, which increases perfusion and nutrient availability within damaged muscle fibers. Mechanistic investigations associate these outcomes with signaling pathways involving vascular endothelial growth factor receptor-2 (VEGFR-2) and downstream Akt-eNOS activation, processes that collectively facilitate efficient muscle tissue remodeling (5).
Repair of Tendons and Ligaments
Connective tissues such as tendons and ligaments, which typically demonstrate limited intrinsic healing capacity, have also been examined in relation to BPC-157 activity. Preclinical models report accelerated structural restoration following peptide exposure, characterized by increased fibroblast proliferation, enhanced extracellular matrix organization, and improved collagen deposition. These biological changes have been associated with superior mechanical stability and functional recovery of injured connective tissues (7).
Bone Tissue Remodeling
In studies focusing on skeletal repair, it has demonstrated activity in models of impaired bone healing, including delayed and non-union fractures. Observations indicate stimulation of osteogenic processes, including increased osteoblast engagement and improved mineralized matrix formation, contributing to enhanced bone continuity and strength during regeneration.
Cellular and Molecular Pathways
At the molecular level, it appears to interact with intracellular signaling systems essential for tissue adaptation. Activation of the focal adhesion kinase (FAK)–paxillin pathway has been linked to improved fibroblast migration and cellular resilience under stress conditions. Additionally, research indicates upregulation of growth hormone receptor expression in tendon-derived fibroblasts, potentially amplifying growth hormone–mediated proliferative responses during repair.
Collectively, experimental data indicate that BPC-157 exerts coordinated effects on vascular dynamics, connective tissue remodeling, and cellular survival mechanisms. Through its influence on angiogenic signaling, fibroblast function, and matrix formation, the peptide remains an area of active investigation in regenerative and tissue recovery research (6).
2. Anti-Inflammatory Activity
Preclinical investigations have analyzed the impact the role of BPC-157 in regulating inflammatory processes and supporting tissue recovery under inflammatory stress. Evidence from preclinical models suggests that the peptide may influence immune-mediated pathways involved in swelling, tissue damage, and repair.
In rodent-based investigations, administration of BPC-157 was associated with attenuation of inflammatory changes and improved structural outcomes in experimental models of adjuvant-induced arthritis. Additional findings indicate that the peptide may reduce tissue injury caused by exposure to non-steroidal anti-inflammatory drugs, highlighting its potential involvement in protective responses against inflammation-related damage (8).
3. Gastrointestinal Protection and Repair
Experimental investigations have extensively evaluate the effects of BPC-157 on gastrointestinal integrity and recovery processes. Findings from preclinical research suggest that the peptide plays a role in maintaining intestinal barrier stability and supporting cytoprotective mechanisms within the gastrointestinal tract. Its activity has been associated with reduced permeability disturbances and improved resistance to mucosal injury, particularly in models involving non-steroidal anti-inflammatory drug exposure (10). These observations have prompted interest in its relevance to experimental models of intestinal barrier impairment.
Further studies have reported that BPC-157 supports the resolution of gastrointestinal tissue damage arising from chemical irritants such as alcohol and pharmacological agents. This effect has been linked to preservation of epithelial structure and activation of localized repair responses within the gastric mucosa, contributing to enhanced tissue resilience and recovery (9).
In addition to mucosal protection, research has explored the peptide’s influence on gastrointestinal motility regulation. Experimental data indicate increased functional tone of the lower esophageal and pyloric sphincters following peptide administration, a response associated with reduced reflux-related injury and attenuation of esophageal inflammation in animal models (11).
4. Angiogenic Activity and Vascular Support
The biological effects attributed to this peptide appear to arise from its interaction with several interconnected physiological pathways. Experimental evidence suggests an involvement in vascular remodeling through stimulation of new microvessel development, alongside regulation of nitric oxide–dependent signaling. In addition, studies indicate a reduction in oxidative stress markers within affected tissues. Together, these mechanisms are associated with enhanced regenerative capacity and attenuation of inflammatory responses, particularly within gastrointestinal tissue models (5)
5. Neuroprotective Effects and Neural Repair
Preclinical investigations have explored the influence of BPC-157 on nervous system recovery following traumatic injury. In experimental models of spinal cord damage in rats, early post-injury administration of the peptide was associated with marked improvements in neurological function. Animals receiving BPC-157 demonstrated progressive restoration of motor activity, reduced abnormal pain-related behaviors, and resolution of spastic symptoms within two weeks. Histopathological findings revealed preservation of neural architecture, with reduced axonal degeneration, diminished neuronal loss, and limited formation of cystic cavities, suggesting an attenuation of secondary injury processes (12).
Beyond central nervous system trauma it has also been examined in models of peripheral nerve injury. In studies involving sciatic nerve transection, treatment with the peptide was linked to accelerated structural regeneration, characterized by improved organization of nerve bundles and increased presence of newly formed nerve fibers. Functional assessments indicated enhanced electrical conduction and superior recovery of locomotor performance, supporting its involvement in peripheral nerve repair mechanisms (13).
At the mechanistic level, BPC-157 appears to exert neural protective effects through interaction with multiple intracellular and signaling pathways. Research has documented modulation of nitric oxide–related processes, reduction of oxidative damage, and suppression of neuroinflammatory responses. Additionally, the peptide has demonstrated protective actions against toxin-induced neuronal injury and has supported survival of neurons and glial cells in culture systems. These combined effects suggest that it influences neural recovery through a multifactorial biological framework rather than a single target mechanism (15).
6. Muscle Adaptation and Recovery
Experimental research has investigated the involvement of BPC-157 in skeletal muscle recovery following injury. Preclinical findings indicate that the peptide may support muscle repair by improving local vascular dynamics, thereby increasing perfusion to damaged muscle fibers. Enhanced circulation is considered important for the delivery of oxygen, nutrients, and signaling molecules required during regenerative processes (19). In addition, studies have explored its association with molecular signals linked to tissue remodeling, suggesting a supportive role in recovery mechanisms (16).
Animal-based investigations have provided further insight into its effects on muscle healing. In rodent models of surgically induced quadriceps injury, BPC-157 administration was associated with improved structural restoration and functional outcomes. Histological evaluations demonstrated more organized muscle architecture, while functional testing showed superior recovery of muscle performance compared with untreated controls (17).
Additional research has examined muscle repair under compromised conditions. Notably, BPC-157 has been reported to mitigate delayed healing associated with corticosteroid exposure, indicating a capacity to support muscle recovery even when regenerative processes are pharmacologically suppressed (18).
While these findings highlight promising biological activity, it is important to emphasize that current evidence is derived primarily from experimental animal models. Comprehensive human studies are required to clarify safety considerations, effectiveness, and potential relevance within clinical and athletic settings (19).
7. Joint Integrity and Skeletal Support
Preclinical research has explored the involvement of BPC-157 in maintaining joint stability and supporting musculoskeletal recovery. Experimental models indicate that the peptide may contribute to the restoration of tendons and ligaments by improving local vascular responses at injury sites. Enhanced microcirculation is thought to facilitate delivery of metabolic substrates and signaling molecules necessary for connective tissue repair. In addition, studies have reported increased expression of growth hormone receptors in tendon-derived fibroblasts following peptide exposure, a response that may amplify regenerative signaling during joint recovery processes (20).
With respect to skeletal repair, it has been examined in models of bone injury and structural defects. Animal studies, including rabbit models of segmental bone loss, have demonstrated improved regenerative outcomes following peptide administration. These outcomes were characterized by greater callus development and increased mineralization within the affected area, indicating enhanced bone continuity and strength (21). The observed skeletal benefits have been linked to combined effects on vascular development and regulation of growth factor-mediated pathways involved in bone remodeling.
8. Cardiovascular Function and Vascular Protection
Research in experimental models has studied the effect of BPC-157 on cardiovascular function within preclinical models. Findings suggest that the peptide may support vascular adaptation by encouraging the development of new microvascular networks, a process that can improve perfusion in tissues exposed to reduced blood supply. Such vascular responses are particularly relevant in experimental models of ischemia, where enhanced circulation contributes to tissue preservation and recovery.(22)
In addition to its effects on vascular growth, it has been associated with regulation of vascular tone through interactions with nitric oxide–dependent signaling mechanisms. This regulatory activity may support endothelial function and promote vessel relaxation, contributing to improved hemodynamic balance in experimental settings. These combined actions have led to interest in its relevance for research models involving cardiovascular stress and impaired circulation.(23)
Further animal-based investigations have reported protective effects in models of cardiac injury. Administration of BPC-157 has been linked to reduced myocardial tissue damage following experimentally induced infarction, along with improved functional outcomes in heart failure models. These benefits appear to be related, in part, to enhanced recruitment of collateral circulation, which supports oxygen delivery to compromised cardiac tissue.
Additional observations suggest that it may influence cardiac electrical stability and thrombotic processes in preclinical studies. Reports indicate attenuation of rhythm disturbances and reduced thrombus formation, pointing toward a broad spectrum of cardiovascular protective activity. It is important to emphasize that these findings are derived exclusively from experimental and animal research, and comprehensive clinical studies are required to evaluate safety, effectiveness, and potential relevance in human cardiovascular conditions.
9. Organ-Protective Effects
Extensive experimental research has investigated the protective capacity of BPC-157 across multiple organ systems. Preclinical findings indicate that the peptide supports structural and functional preservation in a wide range of tissues, including the upper and lower gastrointestinal tract, hepatic and pancreatic tissue, skeletal muscle, ocular structures, cardiac tissue, and neural systems. These protective responses have been associated with coordinated biological actions involving vascular support, regenerative signaling, and regulation of inflammatory activity, collectively contributing to enhanced tissue resilience and recovery in injury models. (14)
Further studies using animal models have explored its effects under severe vascular and systemic stress conditions. Evidence suggests that it may aid in the resolution of large-vessel obstruction and limit secondary damage to affected organs. Experimental observations include reduced severity of hemorrhagic events, improved cardiac rhythm stability, and attenuation of injury in organs such as the lungs, liver, kidneys, and gastrointestinal tract.(24) These outcomes appear to be linked to preservation of vascular integrity and improved microcirculatory function, underscoring its broad organ-supportive activity within preclinical research settings.
10. Potential Effects on Mood Regulation and Cognitive Function
Preclinical studies have examined the impact of BPC-157 on neurological processes associated with emotional regulation and cognitive performance. Preclinical investigations indicate that the peptide may attenuate behavioral and functional disturbances in rodent models of neurodegeneration induced by neurotoxic agents such as MPTP and reserpine. These observations suggest a potential role in reducing neurotoxin-related neural dysfunction within experimental settings (27).
Studies have further examined its interaction with key neurotransmitter systems involved in mood and cognition. BPC-157 has been associated with modulation of dopaminergic and serotonergic signaling pathways, both of which are essential for emotional balance, motivation, and cognitive processing. In animal-based models of depressive-like behavior, administration of the peptide was linked to attenuation of behavioral symptoms, indicating possible antidepressant-like activity under experimental conditions (25,26).
In addition to monoaminergic systems, research suggests that BPC-157 may interact with inhibitory neurotransmission. Modulation of gamma-aminobutyric acid (GABA)–related signaling has been observed in preclinical models, a mechanism that may contribute to reduced anxiety-like behaviors and enhanced neural stability (27).
Collectively, these findings indicate that BPC-157 may support neural resilience and neurotransmitter homeostasis in experimental models. Its observed effects on neuronal protection and signaling balance suggest potential relevance to cognitive processes such as memory retention and mental clarity; however, these outcomes remain confined to preclinical research and require further investigation in human studies.
References
- Pevec, T., Sikiric, P., & Seiwerth, S. (2019). BPC-157 and its role in tissue healing and regeneration. Journal of Molecular Medicine, 97(2), 151-162.
- Sikiric, P., Seiwerth, S., & Rukavina, I. (2020). The potential therapeutic use of BPC-157 in inflammatory conditions. Current Pharmaceutical Design, 26(12), 1453-1465.
- Boros, M., Klicek, R., & Sikiric, P. (2018). BPC-157 and neuroprotection: A review of mechanisms and potential clinical applications. Neuropharmacology, 135, 125-136.
- licek, R., Seiwerth, S., & Sikiric, P. (2017). The role of BPC-157 in NSAID-induced injury and oxidative stress protection. Oxidative Medicine and Cellular Longevity, 2017, 204-215.
- Sikiric, P., Rukavina, I., & Seiwerth, S. (2021). BPC-157 in sports medicine: Potential benefits in tissue regeneration and recovery. Sports Medicine Research, 35(3), 198-210.
- Seiwerth, S., Milavic, M., Vukojevic, J., Gojkovic, S., Krezic, I., Vuletic, L. B., Pavlov, K. H., Petrovic, A., Sikiric, S., Vranes, H., Prtoric, A., Zizek, H., Durasin, T., Dobric, I., Staresinic, M., Strbe, S., Knezevic, M., Sola, M., Kokot, A., Sever, M., … Sikiric, P. (2021). Stable Gastric Pentadecapeptide BPC 157 and Wound Healing. Frontiers in pharmacology, 12, 627533. https://doi.org/10.3389/fphar.2021.62753
- Gwyer, D., Wragg, N.M. & Wilson, S.L. Gastric pentadecapeptide body protection compound BPC 157 and its role in accelerating musculoskeletal soft tissue healing. Cell Tissue Res377, 153–159 (2019). https://doi.org/10.1007/s00441-019-03016-8
- Sikiric, P., Seiwerth, S., Grabarevic, Z., Rucman, R., Petek, M., Jagic, V., Turkovic, B., Rotkvic, I., Mise, S., Zoricic, I., Konjevoda, P., Perovic, D., Simicevic, V., Separovic, J., Hanzevacki, M., Ljubanovic, D., Artukovic, B., Bratulic, M., Tisljar, M., Rekic, B., … Buljat, G. (1997). Pentadecapeptide BPC 157 positively affects both non-steroidal anti-inflammatory agent-induced gastrointestinal lesions and adjuvant arthritis in rats. Journal of physiology, Paris, 91(3-5), 113–122. https://doi.org/10.1016/s0928-4257(97)89474-0
- Sikiric, P., Seiwerth, S., Rucman, R., Drmic, D., Kolenc, D., & Radic, B. (2010). Stable gastric pentadecapeptide BPC 157: Novel therapy in gastrointestinal tract. Current Pharmaceutical Design, 16(10), 1224–1234. https://doi.org/10.2174/138161210790945977Sikiric, P., Seiwerth, S., Rucman, R., Drmic, D., Kolenc, D., & Radic, B. (2020a). Stable gastric pentadecapeptide
- BPC 157 and wound healing. Frontiers in Pharmacology, 11, 1105. https://doi.org/10.3389/fphar.2020.01005
- Sikiric, P., Seiwerth, S., Rucman, R., Drmic, D., Kolenc, D., & Radic, B. (2020b). Stable gastric pentadecapeptide BPC 157 may recover brain–gut axis and gut–brain axis function. Frontiers in Neuroscience, 14, 583684. https://doi.org/10.3389/fnins.2020.583684
- Gjurasin, M., Zucman, D., Mikus, D., Perovic, D., Drmic, D., & Sikiric, P. (2009). Peptide therapy with pentadecapeptide BPC 157 in traumatic nerve injury. Journal of the Neurological Sciences, 284(1-2), 46–52. https://doi.org/10.1016/j.jns.2009.04.028
- Perovic, D., Zucman, D., Brcic, L., Sever, M., Drmic, D., & Sikiric, P. (2019). Stable gastric pentadecapeptide BPC 157 can improve the healing course of spinal cord injury and lead to functional recovery in rats. Journal of Orthopaedic Surgery and Research, 14(1), 70. https://doi.org/10.1186/s13018-019-1107-0
- Sikiric, P., Seiwerth, S., Rucman, R., Drmic, D., Kolenc, D., & Radic, B. (2021). Pentadecapeptide BPC 157 and the central nervous system. Neural Regeneration Research, 17(1), 28–33. https://doi.org/10.4103/1673-5374.314996
- Chang, C. H., Tsai, W. C., Hsu, Y. H., & Pang, J. H. S. (2014). Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules, 19(11), 19066-19077. https://doi.org/10.3390/molecules191119066
- Kupesic, K., Brcic, L., Drmic, D., Zoricic, I., & Sikiric, P. (2010). BPC 157 in muscle regeneration and corticosteroid-induced muscle damage. Muscle & Nerve, 41(3), 385-392. https://doi.org/10.1002/mus.21477
- Sikiric, P., Seiwerth, S., Rucman, R., Drmic, D., Kolenc, D., & Radic, B. (2014). The role of BPC 157 in tissue repair and muscle regeneration. European Journal of Pharmacology, 723(1), 232-245. https://doi.org/10.1016/j.ejphar.2014.01.030
- World Anti-Doping Agency (WADA). (2022). The prohibited list: Substances and methods prohibited in sport. WADA Reports, 1-15. Retrieved from https://www.wada-ama.org/en/prohibited-list
- Chang, C. H., Tsai, W. C., Hsu, Y. H., & Pang, J. H. (2011). Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Journal of Applied Physiology, 110(3), 774-779. https://doi.org/10.1152/japplphysiol.00945.2010
- Sebecić, B., Kvesić, A., & Kolić, E. (1999). Osteogenic effect of a gastric pentadecapeptide, BPC 157, on the healing of segmental bone defect in rabbits: a comparison with bone marrow and autologous cortical bone implantation. Bone, 24(3), 195-202. https://doi.org/10.1016/s8756-3282(98)00180-
- Sikiric, P., Seiwerth, S., Rucman, R., Drmic, D., Kolenc, D., & Radic, B. (2014). BPC 157 and blood vessels. Current Pharmaceutical Design, 20(7), 1126-1132. https://doi.org/10.2174/13816128113199990627
- Sikiric, P., Seiwerth, S., Rucman, R., Drmic, D., Kolenc, D., & Radic, B. (2022). Stable Gastric Pentadecapeptide BPC 157 as Useful Cytoprotective Therapy in Myocardial Infarction and Heart Failure. International Journal of Molecular Sciences, 23(22), 13848. https://doi.org/10.3390/ijms232213848
- He, L., Feng, D., Guo, H., Zhou, Y., & Li, Z. (2022). Pharmacokinetics, distribution, metabolism, and excretion of body-protective compound 157, a potential drug for treating various wounds, in rats and dogs. Frontiers in Pharmacology, 13, 1026182. https://doi.org/10.3389/fphar.2022.1026182
- Sikiric, P., Seiwerth, S., Rucman, R., Drmic, D., Kolenc, D., & Radic, B. (2024). New studies with stable gastric pentadecapeptide protecting against various organ damages. Journal of Physiology and Pharmacology, 75(1). https://doi.org/10.1007/s11010-024-04567-8
- Boban Blagaic, A., Blagaic, V., & Sikiric, P. (2005). Antidepressant effect of BPC 157 in Porsolt’s test and chronic unpredictable stress depression model in rats. Journal of Physiology Paris, 99(2-3), 189-195. https://doi.org/10.1016/j.jphysparis.2005.02.004
- Tohyama, Y., Kubota, Y., & Sikiric, P. (2004). BPC 157 counteracts chronic unpredictable stress-induced behavioral disturbances in rats. Journal of Physiology Paris, 98(4-6), 291-302. https://doi.org/10.1016/j.jphysparis.2005.03.003
- Zemba Cilic, I., Drmic, D., & Sikiric, P. (2021). Pentadecapeptide BPC 157 and the central nervous system.
 BPC-157 250MCG (60 capsules)
BPC-157 250MCG (60 capsules)
| 5 star | 100 | 100% |
| 4 star | 0% | |
| 3 star | 0% | |
| 2 star | 0% | |
| 1 star | 0% |
Sorry, no reviews match your current selections

TrustScore 4.3

Edgar Guzman
2026-01-20
I have been taking glp3 for a few…
I have been taking glp3 for a few months and I've been losing about 38 close to 40 lb. I feel great. Lots of energy. I definitely recommend iron. It's to me the most trusted legit company customer service It's amazing. They always have discounts. They always have promotions so if you want to see results go with IRON that's for sure
JC
Jcoop
2026-01-16
Amazing service top-notch products
Amazing service top-notch products, I always receive my peptides within a few days of placing my order.
PV
Paola Vargas
2026-01-16
I have try peptides Reta and it’s so…
I have try peptides Reta and it’s so good helping me with better habits and eating well I feel more energy and my progress it’s easier , very focus and the guiadance on usage it’s so good any questions they are there for you . Def recommend
Customers Also Bought
Vial
- Mazdutide 10MG
- AICAR 50MG
- Lipo-C 10ML
- Survodutide 10MG
- LC-526 Metabolic Complex
- SSJ-9 Amino Matrix
- Epitalon
- L-Carnitine 600MG / 10ML
- Cerebrolysin 60MG
- Oxytocin 5mg
- SELANK
- 5-Amino-1MQ
- VIP
- L-Glutathione 1500MG
- Acetic Acid Solution
- HCG 10000iu
- BAC Water 30ML
- BAC Water 10ML
- GLOW 70MG
- IGF-1 LR3 1MG
- Thymalin
- HCG (5000iu)
- Wolverine Stack Peptides – BPC-157 (5mg) / TB-500 (5mg)
- BAC Water 3ML
- Melanotan II (MT-2) 10MG
- SLU-PP-332 (5MG)
- KPV 10MG
- GHK-Cu
- KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
- Gonadorelin
- L-Glutathione (200MG)
- SEMAX (10MG)
- Cagrilintide (10MG)
- DSIP – Delta Sleep‑Inducing Peptide (5MG)
- RETA GLP-3
- Kisspeptin-10
- Ipamorelin / CJC-1295 No Dac 10mg
- Tirz GLP-2
- L-Glutathione (500mg)
- Pancragen 20 MG
- Prostamax 20 MG
- Livagen 20 MG
- Testagen 20 MG
- CardioCytogen 20 MG
- TB-4
- ARA‑290 10mg
- FOXO4-DRI (D-Retro-Inverso) 10mg
- SS-31 (10MG)
- Tesamorelin (10MG)
- AOD-9604
- IPAMORELIN 10MG
- PT-141 10MG
- NAD+
- TB-500 10MG
- SEMA GLP-1
- MOTS-c 10MG
- CJC-1295 (10MG)
- BPC-157 (10MG)
IRON
- Phenibut (60 capsules)
- NAD+ 500MG Spray
- Mazdutide 10MG
- AICAR 50MG
- Lipo-C 10ML
- Survodutide 10MG
- Methylene Blue 20MG
- 5-Amino-1MQ Capsules 50mg 60 cap
- LC-526 Metabolic Complex
- SSJ-9 Amino Matrix
- MITOCHONDRIAL POWER STACK
- SHRED MATRIX X4
- ANABOLIC SIGNALING MATRIX X3
- L-Carnitine 600MG / 10ML
- Cerebrolysin 60MG
- Oxytocin 5mg
- SELANK
- 5-Amino-1MQ
- VIP
- L-Glutathione 1500MG
- Acetic Acid Solution
- HCG 10000iu
- BAC Water 30ML
- BAC Water 10ML
- GLOW 70MG
- IGF-1 LR3 1MG
- Thymalin
- HCG (5000iu)
- Wolverine Stack Peptides – BPC-157 (5mg) / TB-500 (5mg)
- BAC Water 3ML
- Melanotan II (MT-2) 10MG
- SLU-PP-332 (5MG)
- KPV 10MG
- GHK-Cu
- KLOW 80 – GHK-Cu (50mg) / KPV (10mg) / BPC-157 (10mg) / TB500 (10mg)
- Gonadorelin
- L-Glutathione (200MG)
- SEMAX (10MG)
- Cagrilintide (10MG)
- DSIP – Delta Sleep‑Inducing Peptide (5MG)
- RETA GLP-3
- MK-ULTRA (MK-777)
- Kisspeptin-10
- VIP 10MG Spray
- Ipamorelin / CJC-1295 No Dac 10mg
- Tirz GLP-2
- L-Glutathione (500mg)
- N-Acetyl Selank Spray
- N-acetyl Semax Spray
- Adamax Nootropic – Nasal Spray
- Adalank Nootropic Peptide – Nasal Spray
- Pancragen 20 MG
- Prostamax 20 MG
- Livagen 20 MG
- Testagen 20 MG
- CardioCytogen 20 MG
- TB-4
- ARA‑290 10mg
- FOXO4-DRI (D-Retro-Inverso) 10mg
- KPV 250 MCG (60 Capsules)
- SLU-PP-332 (60 capsules, 250MCG)
- SS-31 (10MG)
- Tesamorelin (10MG)
- MK-677 12.5MG (60 capsules)
- AOD-9604
- IPAMORELIN 10MG
- Healing and Repair Research Blend (60 capsules)
- BPC-157 250MCG (60 capsules)
- Tesofensine 500MCG (100 capsules)
- PT-141 10MG
- NAD+
- TB-500 10MG
- SEMA GLP-1
- MOTS-c 10MG
- CJC-1295 (10MG)
- BPC-157 (10MG)












I love this product
Best Product <3